Within industries demanding high reliability such as defense and medical technologies, documenting an incredible amount of detail throughout the manufacturing process is essential to regulatory compliance and product quality. Specifically, in the medical design and manufacturing industry, stringent FDA traceability requirements are part of the business—vital for protecting lives, but difficult and expensive to manage.
Benchmark developed a custom traceability system for the manufacture of FDA Class 3 Medical Devices and other high-reliability products. Originally created to simply track defects and component batch lots, over the course of two decades it has matured and grown to encompass activities well beyond those humble beginnings. The Process Feedback System, branded PFS, is Benchmark's proprietary, global manufacturing execution system, now identified as industry best practice. Being a single-application solution enables efficiencies in data sharing, customer communications, project team collaboration, and product continuity of supply risk mitigation. Customers can directly access product data reports increasing transparency, collaboration, and providing customers with the information and peace of mind they need to focus on their core competencies when working with a contract manufacturer.
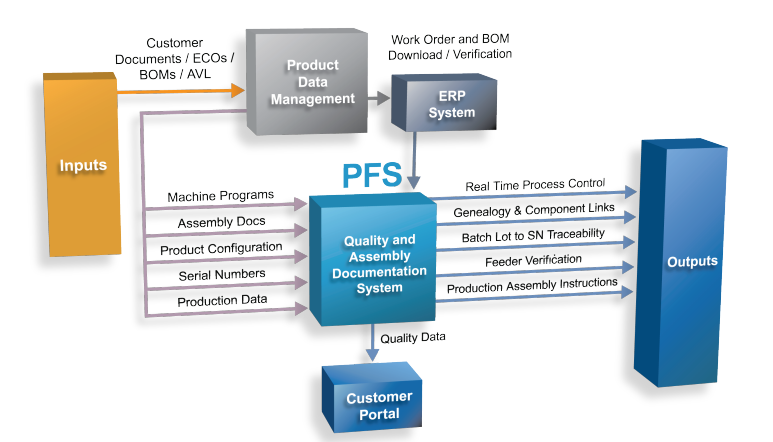
In addition to providing product assembly genealogy—from the finished system-level product all the way back to purchased component manufacturing lot data used on printed circuit board assemblies—the system also:
- Completely paperless
- Serves as the product assembly work instruction media, supporting photo and short video utilization for improved assembly process instruction detail
- Deployed on PCBA and System Integration assembly processes, linking the two together
- Facilitates New Product Introduction (NPI) and sustaining task assignment
- Contains automated work order release verification processes
- Provides real-time process control with automated shutdown mechanisms
- Ensures product is assembled in the proper order and that it meets all required assembly, inspection, and test acceptance criteria
- Integrates worker training records, allowing only trained individuals to access work instructions and enter data
- Utilizes data masking to ensure only approved materials and tooling are used during the assembly process
- Maintains entire product assembly history for return or upgrade programs
- Serves as an extension to the change management process to allow for flow down and regulated change approval workflows
- Integrated with Enterprise Resource Planning (ERP) and process assembly, test, and inspection equipment platforms
- Provides a web-based access point for customers to review their process performance data, WIP status reports, and assembly history
A system like PFS offers four key benefits. First, a strict control system offers better product yields facilitated through real-time process control. The drive towards continuous process improvement (through process control) starts with accurate data collection—PFS collects data at every assembly, inspection, and test gate. This information provides a cache of data associated with who performed the operation, defect type, as-built component lot, repaired component lot, and timestamps of all scans. This data is automatically monitored against pre-defined process control criteria, forcing process, and assembly conformance. It can further be analyzed through a number of standard PFS reports, or exported for analysis in tools such as Minitab or Excel, looking for data trends or links.
Second, operating costs are controlled as a result of direct engagement between PFS and inspection and test platforms. Complementary to the automated process shutdown features, these linkages ensure accurate data entry from critical product performance acceptance gates, which help to reduce product returns and assembly labor.
Third, integrating data collection throughout the process and managing the data well fosters better regulatory agency relations as a result of near-immediate retrieval of product build history reports (Design History Record, or DHR). Features such as regulatory review requirements, product visitation control, and component traceability greatly simplify regulatory inspections. This results in successful inspections. In fact, Benchmark has undergone more than fifteen FDA inspections globally without any 483 observations.
Finally, the system encourages stronger supplier/customer relations due to the web-based customer access portal. When teams are able to transparently review and analyze the same data sets, communications are clear and unimpeded. This direct and timely access can provide the trust needed to drive focus and improvement in the right areas.
As the saying goes, knowledge is power. When it comes to component and product traceability, knowledge is also reduced risk and improved efficiency. By collecting data and applying it throughout the process, PFS gives Benchmark and our customers excellent insight and process control when it matters most.
Ready to see how Benchmark's PFS system can benefit your high-reliability product? Contact us!
