Your device deserves an experienced partner in medical engineering and manufacturing. Since our inception as a manufacturing subsidiary of a medical device company, Benchmark leads the market. Our investments in a global network of sites with comprehensive medical certifications provide you the footprint and expertise you need to get your device to market faster and with less risk throughout its lifecycle.
Your organization requires like-minded partners looking to push the limit of what’s possible. Our factory management systems are designed with FDA regulation in mind, allowing us to set the standard for compliance while creating market-leading innovations in crucial solution areas including connected medical devices, energy delivery systems, fluid management, and more.
With expertise built over four decades and an eye towards partnering with the most disruptive and high-growth companies in the sector, you can be confident Benchmark is constantly developing valuable capabilities for your products now and in the future. Design for Excellence reviews, test development, microelectronics, precision machining, and a suite of market-leading systems and platforms can all be delivered customized to your needs. As your requirements change, you can leverage our global footprint to keep engineering and manufacturing close-to-home or close-to-customers for initial production runs, and then push offshore as volumes increase with a seamless and standardized site transition.
We have dedicated connected device software and hardware technology teams that work with medical device companies to provide them with competitive solutions that are HIPAA-compliant. Benchmark offers extensive expertise in helping customers with human factors and ergonomics, along with award-winning industrial design.

We are the manufacturer of choice for the world's most trusted AED companies, combining manufacturing capabilities in PCB assembly and full system build along with industry-leading test development.
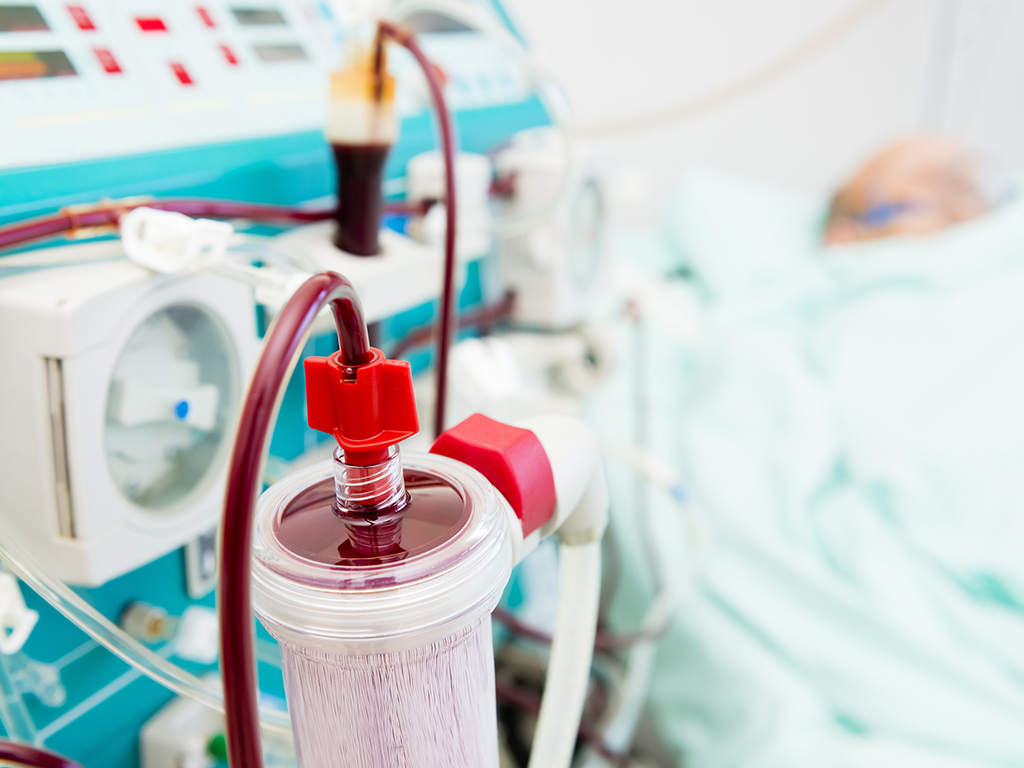
Benchmark's global network of sites designs, manufactures, tests, and provides aftermarket support for the market's leading drug delivery, sterile fluid control, dialysis, and blood management products.
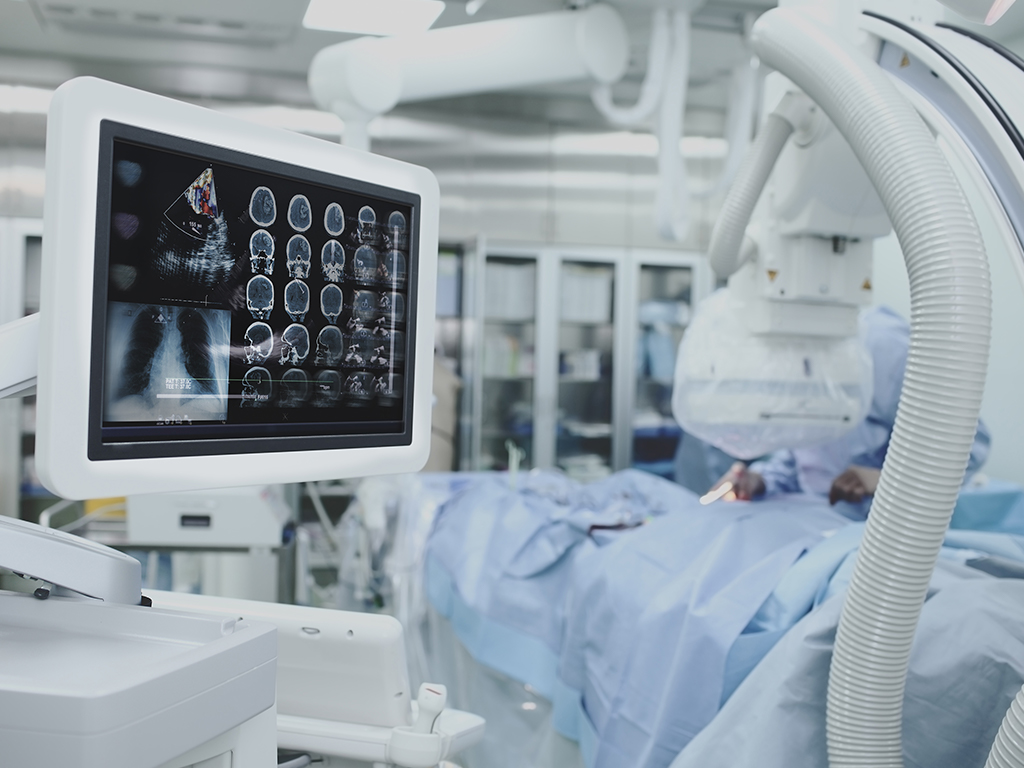
From large Magnetic Resonance Imaging (MRI), Magnetic Rapid Transit (MRT), and Computerized Tomography (CT) systems to the next generation of smaller, mobile, handheld imaging devices, our team ensures precision.

We support innovation in fine-movement robotics with electro-mechanical design and manufacturing, as well as connectivity options that reduce or eliminate lag time for new medical robotics applications.
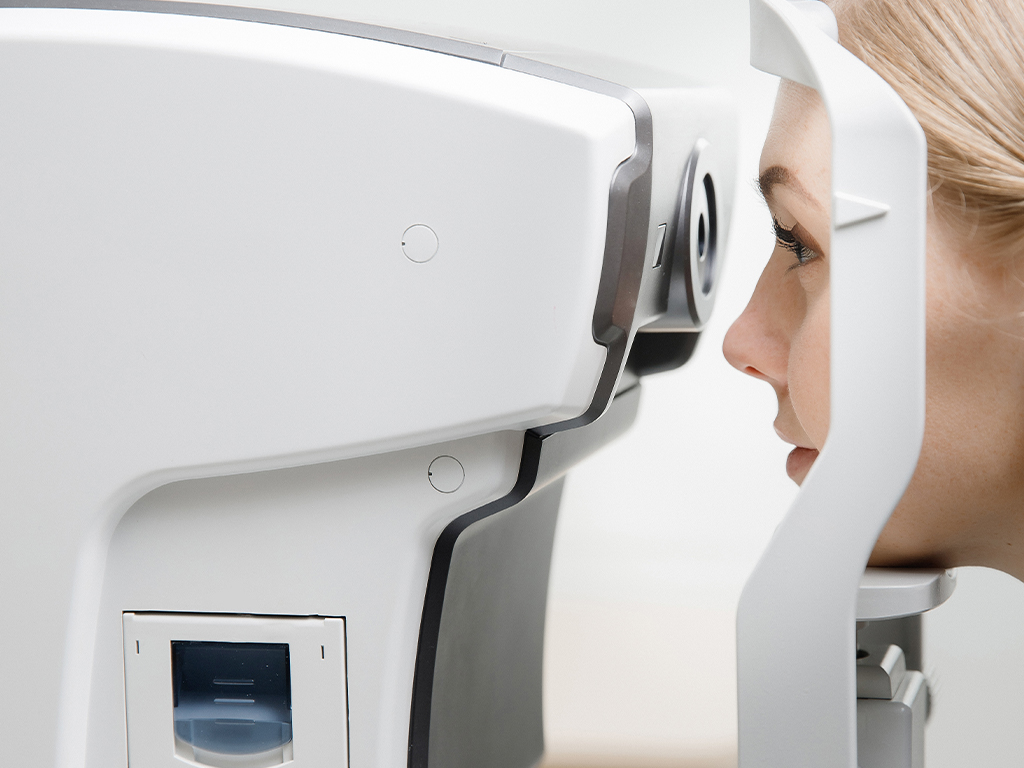
Accurate information saves lives, so you need to know that everything from handheld diagnostic tools to complete diagnostic systems will deliver the highest reliability every time. Benchmark's engineering and manufacturing capabilities support precision optical applications.
Benchmark offers personalized attention on a global scale. In this video, Benchmark Medical Sector Vice President Joe Garcia describes how we support our medical device customers from early concept development through aftermarket services, with processes and capabilities designed to meet the needs of the medical industry.


