New technologies have fundamentally changed the way in vitro diagnostic (IVD) devices look and operate. Today, the use of nanofluidics and micro-electro-mechanical systems (MEMS) has led to designs that are improving patient outcomes, saving lives, and lowering healthcare costs.
The Customer: Abionic
Headquartered in Lausanne, Switzerland, Abionic is committed to delivering rapid, laboratory-quality diagnostic solutions, enabling physicians to make early and appropriate clinical decisions at the point of need.
Abionic’s flagship device, the abioSCOPE® is an innovative IVD point-of-care platform currently commercialized to test for sepsis, allergies, and iron deficiency. The abioSCOPE uses the principles of traditional immunoassay technology as well as patented nanofluidic technology to analyze 14 parameters from a single drop of blood and reduce analysis time from hours to minutes.
The Challenge: Optimizing & Commercializing a Disruptive Technology
The key to addressing the IVD market needs lies within Abionic’s core technology: a novel nanofluidic system that provides on-the-spot biomarker measurement at the point of care. The fundamental invention is a matter of scale; by reducing the sample volume is smaller, and chemical equilibrium is achieved faster. Coupled with state of the art photometric technology, this approach delivers a precise and reliable test that outperforms current industry standards
While Abionic developed a functioning prototype of their second-generation abioSCOPE®, the company required an engineering and manufacturing partner who could help bring their product to market.
To meet clinical requirements and disrupt the market, Abionic needed to reduce sample processing time, increase accuracy, improve the overall quality and usability, while keeping costs low.
In addition to the core immunoassay technology of the platform, the final device had to be able to precisely manipulate the nanofluidic cartridge, a single-use element exchanged in every test, to align it with the onboard optical reader.
To achieve this electro-mechanical challenge, Abionic relied on Benchmark to design a precise, three-dimensional positioning system, in addition to design for manufacturing (DFM) activities, to bring the final device design together into a buildable product.
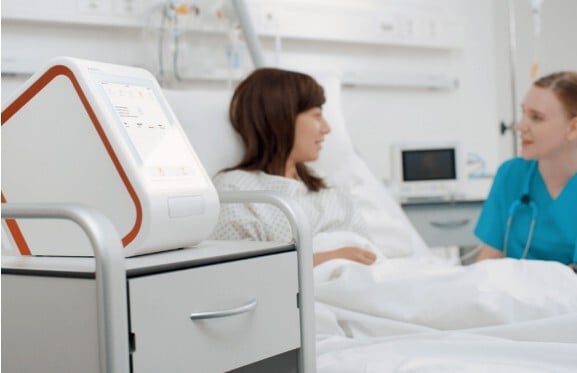 Photo Credit: abioSCOPE® image courtesy of Abionic
Photo Credit: abioSCOPE® image courtesy of Abionic
The Solution: A Collaborative Approach
Abionic partnered with Benchmark to leverage their decades of design engineering expertise and manufacturing experience in the medical device industry. Because of the technical challenges involved in the abioSCOPE® device, it was critical to employ a highly collaborative and iterative approach to the design program.
A great example of Benchmark’s customer-focused approach is in the Creative Workshop that was instrumental in solidifying the partnership between these two companies.
In an intensive two-day workshop, Abionic’s Chief Technical Officer and lead engineers met with Benchmark’s engineering team in an immersive setting to develop a proof-of-concept design aimed at reducing the measurement time of the abioSCOPE®. By defining the product’s requirements and reviewing the existing bill of materials, the two teams were able to clear a path forward and guide the product development towards a successful launch into manufacturing.
The Results: A Disruptive Medical Device at a Competitive Price
By utilizing a joint design approach, Abionic and Benchmark were able to successfully launch the abioSCOPE® into production. Benchmark applied its wide range of capabilities to help Abionic optimize the three-axis precision positioning of the nanofluidic cartridge contained within the abioSCOPE® and to improve the fluorescence detection system. The team accomplished this through high-precision mechanics, an inventive driving solution, and dedicated control software, which was critical to ensure the reproducibility of the positioning process.
Benchmark’s industrial design team also played a significant role in improving the clinical user experience of the finished device. The team redesigned the display and user interface to help healthcare professionals interact with the device.
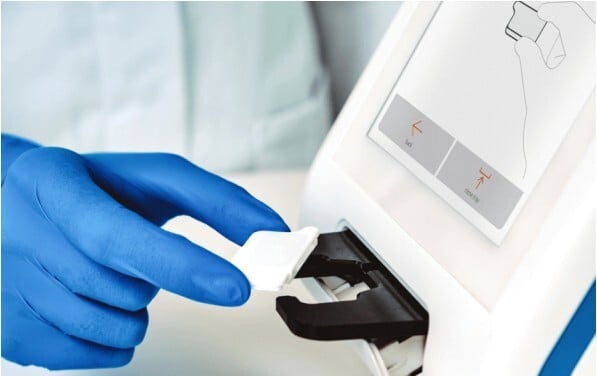 Photo credit: abioSCOPE®'s nanofluidic cartridge image courtesy of ABIONIC.
Photo credit: abioSCOPE®'s nanofluidic cartridge image courtesy of ABIONIC.
To reduce development costs, Benchmark leveraged its global supply chain to make smarter choices in component selection. Benchmark was able to identify lower-cost, off-the-shelf components to replace custom components in the initial prototype.
By applying DFM principles, the team was able to minimize the number of unique parts and cut-down assembly time, further reducing development and manufacturing costs. In tandem, Abionic honed its specialized nanofluidic technology and the immunoassays employed by the device, allowing the platform to perform tests more efficiently for several standard clinical tests. Today, Abionic’s technology is truly a lifesaver when used to diagnosis sepsis, a potentially fatal disease. The abioSCOPE® can identify sepsis in minutes, with most traditional testing taking hours for results.
At Benchmark, we’re redefining what’s possible by helping customers like Abionic develop new clinical testing technology to achieve early disease detection, diagnosis, and targeted intervention. With game changing technologies like Abionic’s, Benchmark is proud to be the trusted partner medical device companies turn to when it matters.
About Benchmark
Benchmark provides comprehensive solutions across the entire product lifecycle, leading through its innovative technology and engineering design services, leveraging its optimized global supply chain and delivering world-class manufacturing services. The industries we serve include commercial aerospace, defense, advanced computing, next-generation telecommunications, complex industrials, medical, and semiconductor capital equipment.